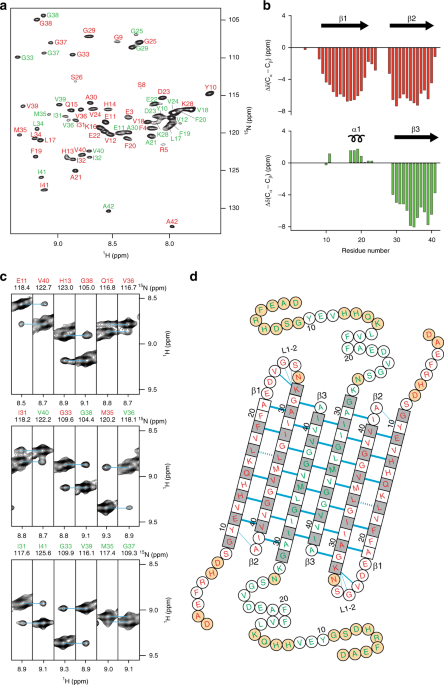
Aβ(1-42) tetramer and octamer structures reveal edge conductivity

Aβ(1-42) tetramer and octamer structures reveal edge conductivity pores as a mechanism for membrane damage

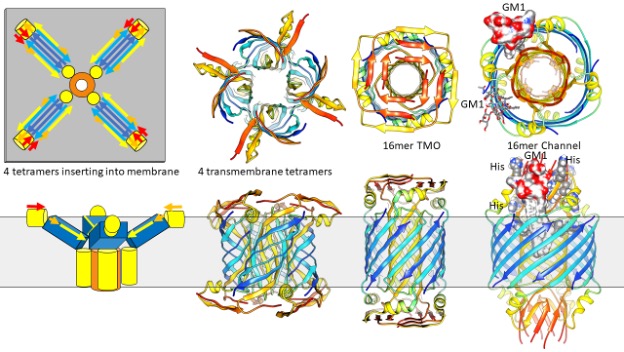
The amyloid concentric β-barrel hypothesis: Models of amyloid beta 42 oligomers and annular protofibrils
Why are the root causes of amyloid-associated diseases so misunderstood and treatments so inadequate?

Exploring amyloid oligomers with peptide model systems - ScienceDirect

Purity and identity of [U-15 N] Aβ42 and [U-2 H, 13 C, 15 N] Aβ42

Structural architecture of amyloid-β oligomers, curvilinear protofibrils and annular assemblies, imaged by cryo-EM and cryo-ET

Structural details of amyloid β oligomers in complex with human prion protein as revealed by solid-state MAS NMR spectroscopy - ScienceDirect
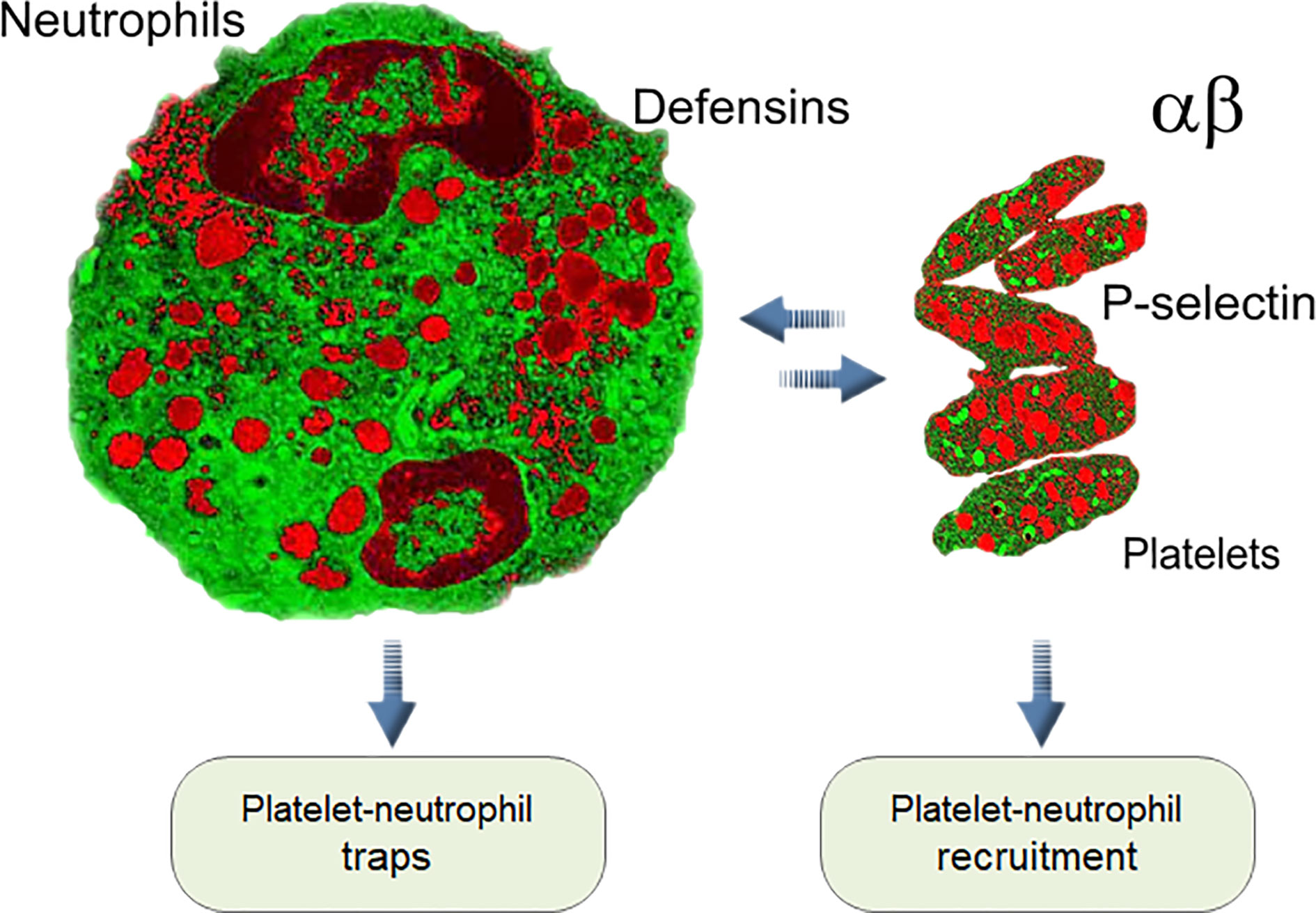
Frontiers On the Role of Platelet-Generated Amyloid Beta Peptides in Certain Amyloidosis Health Complications

A glimpse into the structural properties of α‐synuclein oligomers
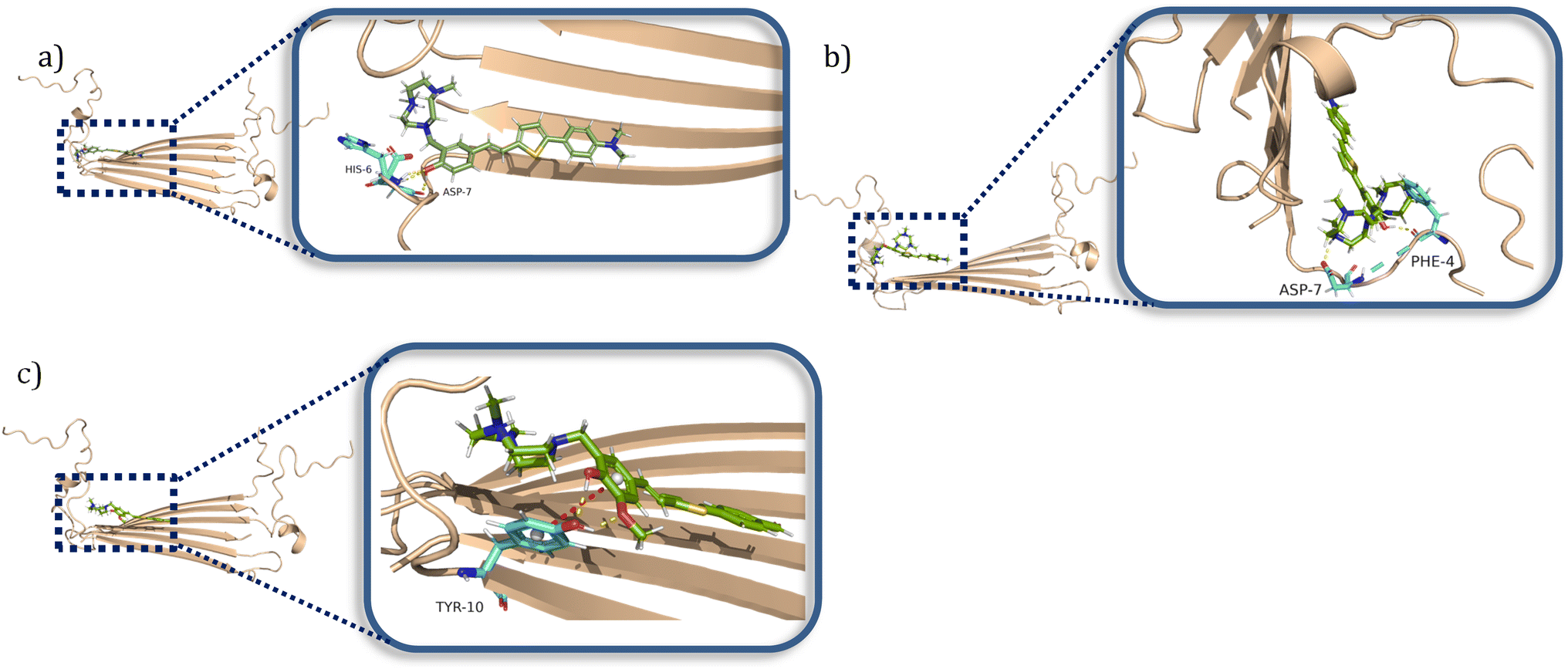
Amphiphilic stilbene derivatives attenuate the neurotoxicity of soluble Aβ 42 oligomers by controlling their interactions with cell membranes - Chemical Science (RSC Publishing) DOI:10.1039/D2SC02654F

The amyloid concentric β-barrel hypothesis: Models of amyloid beta 42 oligomers and annular protofibrils
A new mechanism of toxicity in Alzheimer's disease revealed by the
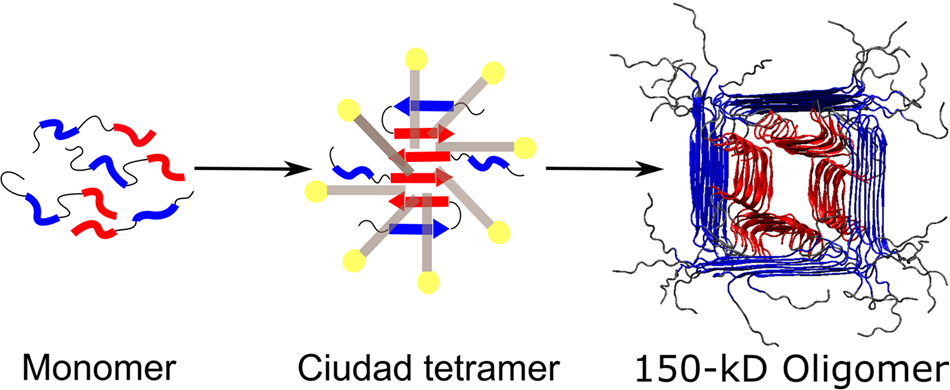
A common pathway for detergent-assisted oligomerization of Aβ42

Structural details of amyloid β oligomers in complex with human









