Compression of a gas due to external pressure and the

4 Entropy changes for a monothermal irreversible process (expansion or

Waldo QUIROZ, Professor (Full), PhD Chemistry

thermodynamics - Are you supposed to use the internal or external pressure for the $pV$ work integral? - Physics Stack Exchange

How to Calculate the Work Done on a Gas Algebraically, Physics

Q. 2 mole of an ideal gas undergoes isothermal compression along three different paths (i) reversible compression from P; = 2 bar and V; = 4 L to P, = 20 bar (

Cristian MERINO RUBILAR, Professor (Assistant)
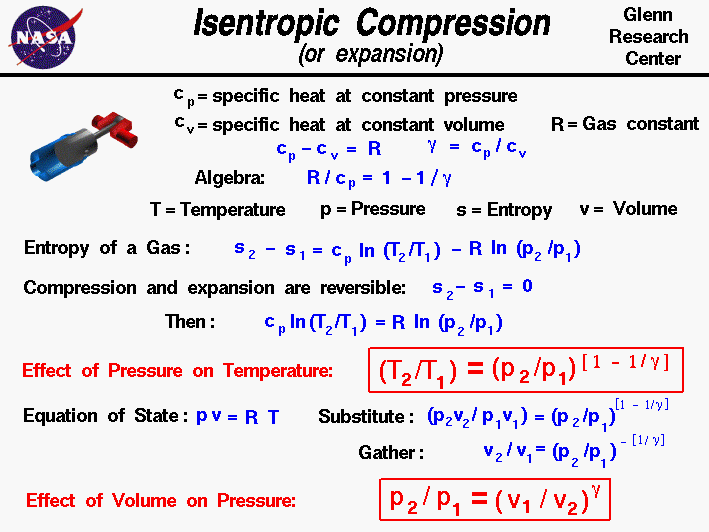
Isentropic Compression or Expansion

Compression of a gas due to external pressure and the corresponding

An ideal gas undergoes isothermal compression from { 5m }^{ 3 } to 1 m^3 against a constant external pressure of 4 N{ m }^{ -2 }. Heat released in this process

An ideal gas undergoes isothermal compression from 5 m3 against a constant external pressure of 4 Nm-2. Heat released in this process is used to increase the temperature of 1 mole of

An ideal gas undergoes isothermal compression from 15 m to 10 m against a constant extemal pressure of 6 N-m2. Heat Q.2 released in this process is used to increase the temperature

Compressed air, Energy Efficiency, Industrial Uses & Safety