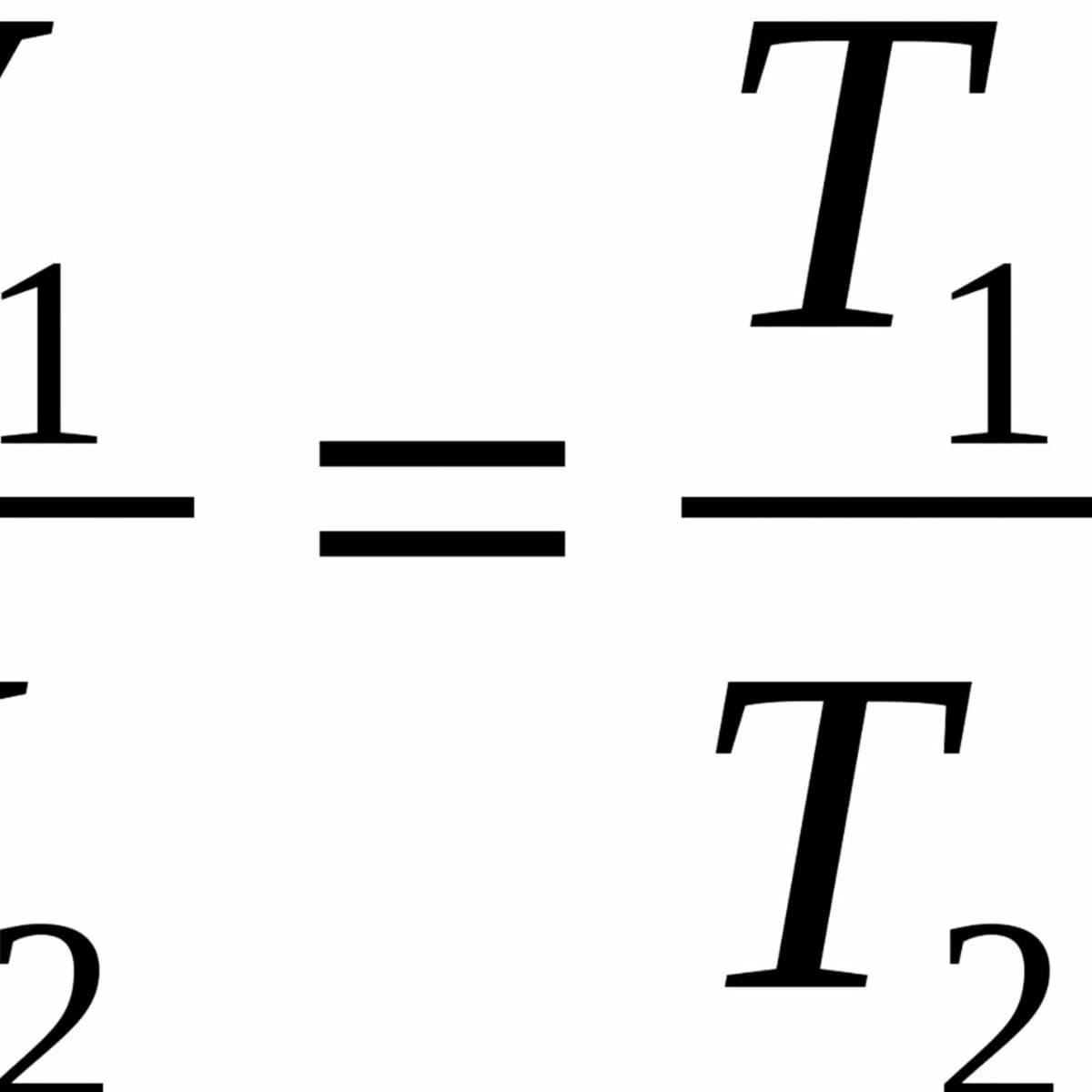kinetic theory - Why doesn't Helium behave as an ideal gas? - Physics Stack Exchange
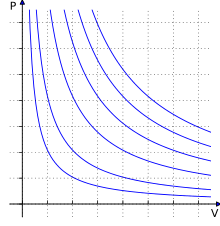
I am a bit confused (might be due to some conceptual misunderstanding) as to why doesn't Helium behave as an ideal gas (it shows a deviation from the $pV$ vs $p$ graph)? (Given the fact that it is

PPT - Gases – Kinetic Theory revisited (assumptions for “ Ideal” Gases) PowerPoint Presentation - ID:4342875
Does specific heat for ideal gas at constant volume depends on temperature? - Quora

statistical mechanics - If liquid and gas are both chaotic states of matter, what's the difference between them on the molecular level? - Physics Stack Exchange
13.4 Kinetic Theory: Atomic and Molecular Explanation of Pressure and Temperature – College Physics
electromagnetism - What phenomena occur in a low voltage arc between copper and graphite electrodes, and why is the result dependent on electrode polarity? - Physics Stack Exchange

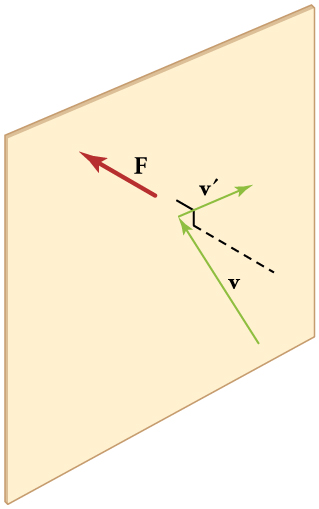
13.4 Kinetic Theory: Atomic and Molecular Explanation of Pressure and Temperature – College Physics: OpenStax
Is helium the noble gas that acts most like an ideal gas? - Quora

thermodynamics - Why it is colder in mountains, at high altitudes? - Physics Stack Exchange

Lectures on Quantum Mechanics. With Problems, Exercises and Solutions [3 ed.] 9783031176340, 9783031176357

download - Enea

statistical mechanics - If liquid and gas are both chaotic states of matter, what's the difference between them on the molecular level? - Physics Stack Exchange

PDF) Physics 516: Electromagnetic Phenomena (Fall 2019)

statistical mechanics - If liquid and gas are both chaotic states of matter, what's the difference between them on the molecular level? - Physics Stack Exchange