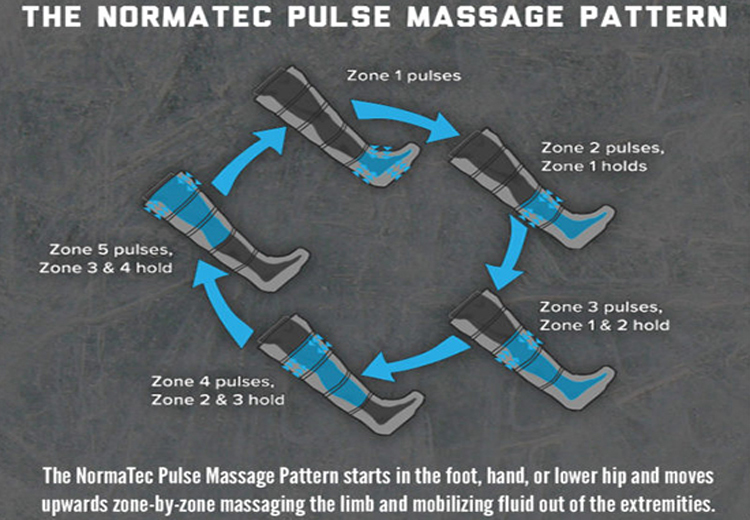
AIROS Medical Receives FDA Clearance to Market New Peristaltic
4.6
(418)
Write Review
More
$ 10.00
In stock
Description
AIROS Medical announces FDA 510k clearance to market the AIROS 8P compression device and Pants garment that treats leg and pelvic swelling.

First Quality NGX-013 - McKesson Medical-Surgical

Compression Wear And Shapewear Market Trend Analysis, And Forecast To 2033
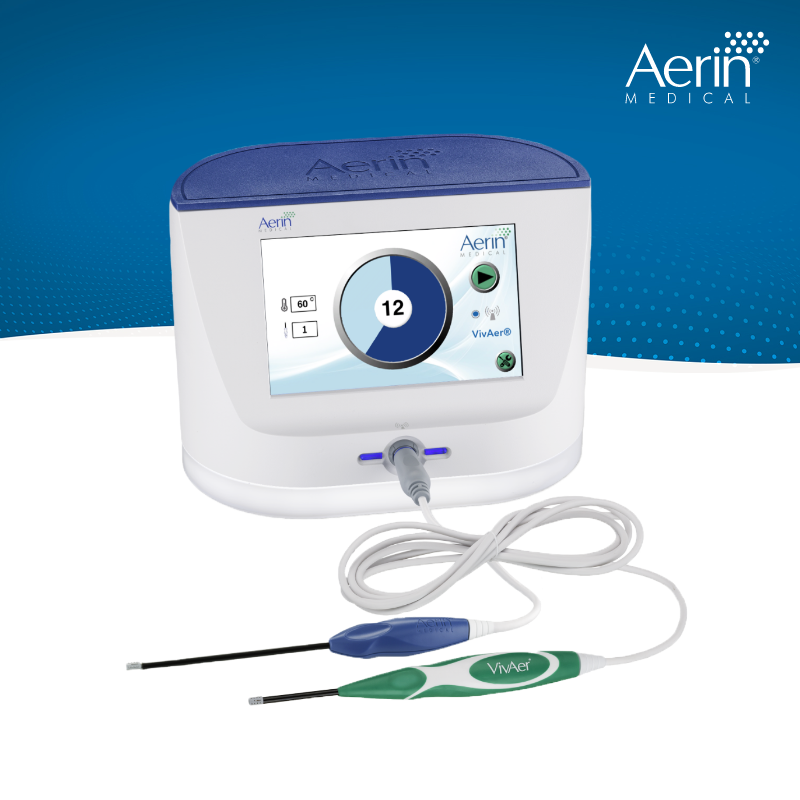
FDA Clearance of Procedure for Chronic Rhinitis

AIROS Medical Granted U.S. Trademark Registration for Company

Airway Clearance System Market – Global Industry Trends and

LungFit PH – P200044

Avanos Medical 2205 - Ballard Turbo CS Adult, 14 FR, T-Piece, 20 EA/CS - CIA Medical

Latest Health News in America

What is a lymphedema pump or pneumatic compression pump?

FDA approves LungFit PH to treat respiratory failure in neonates
You may also like