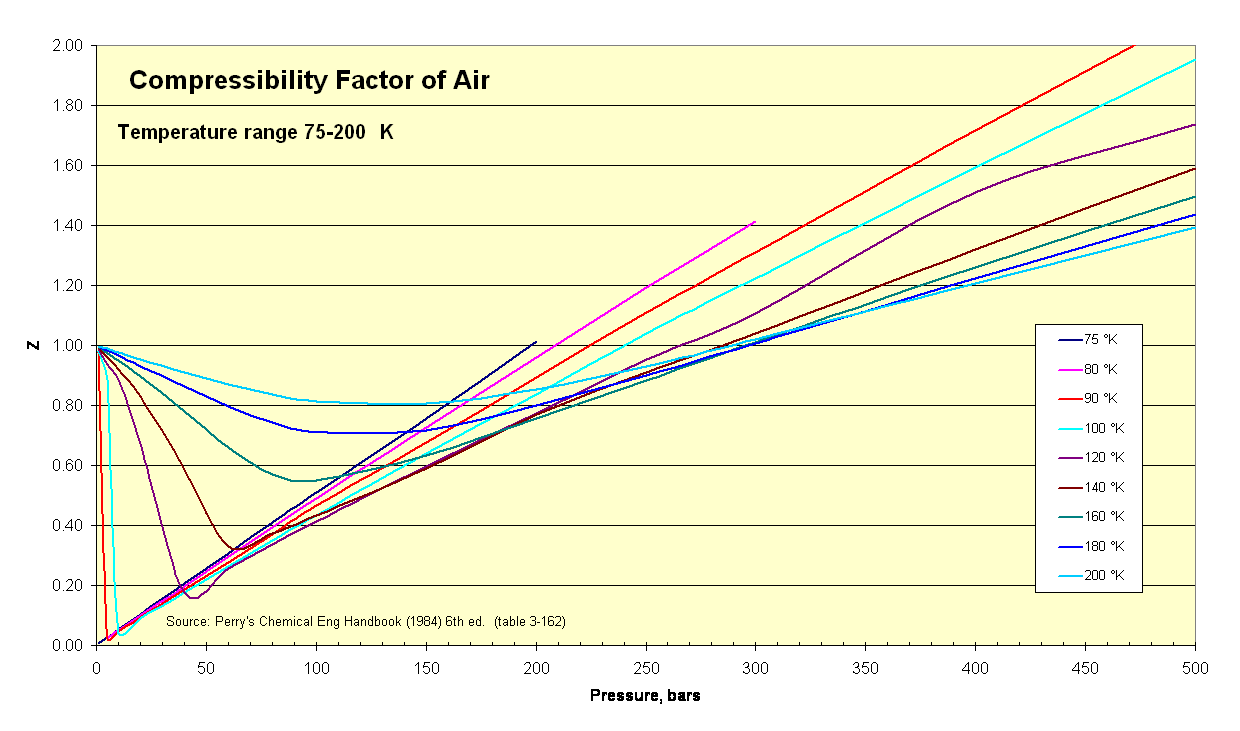
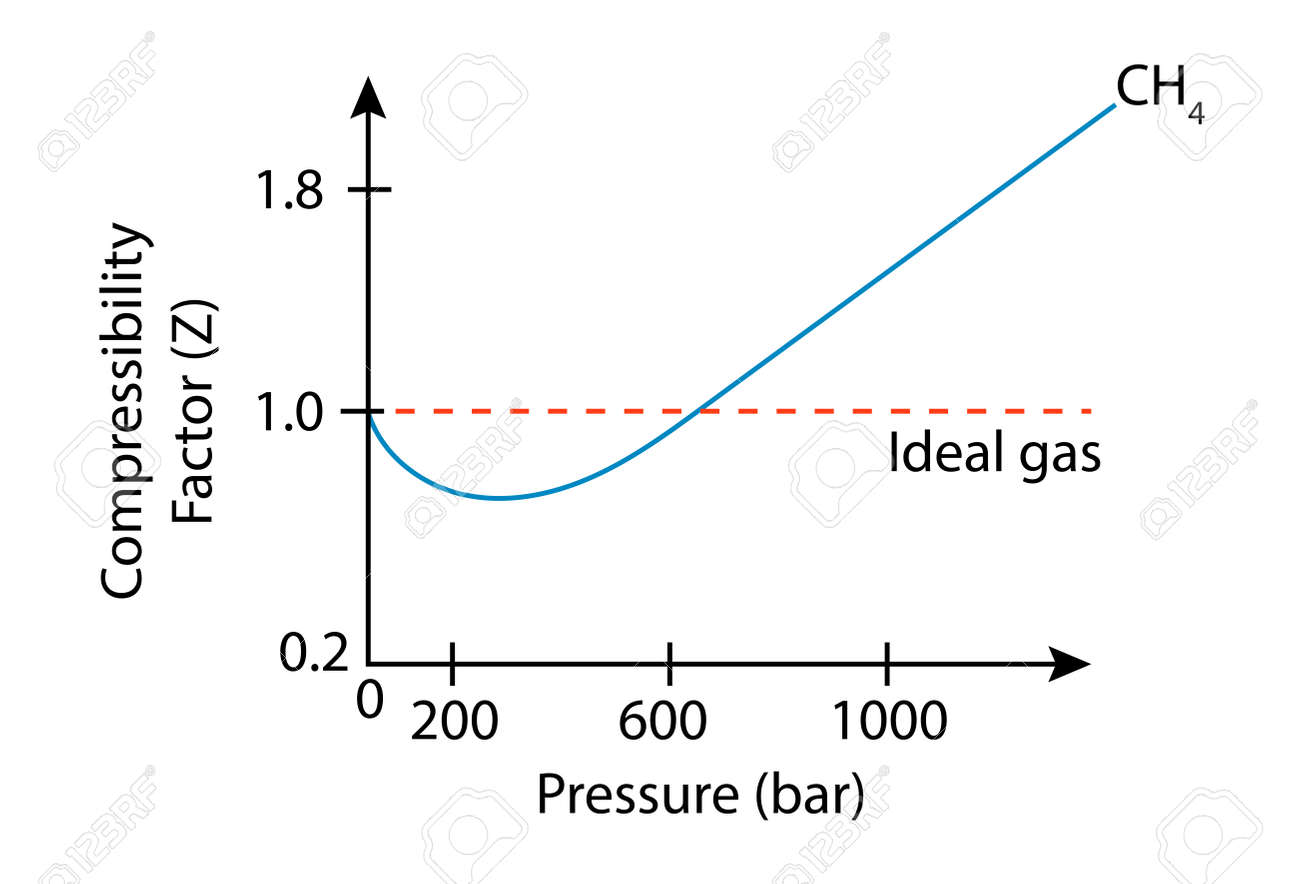
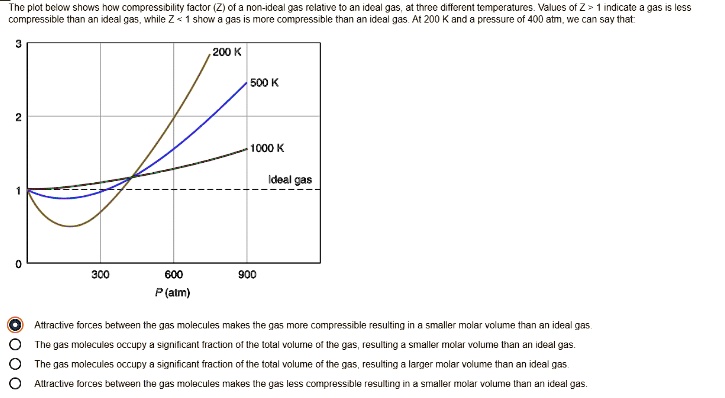
Solved The compressibility factor, Z, can be thought of as a
Answer to Solved The compressibility factor, Z, can be thought of as a
For compressibility factor, Z, which of the following is /are correct?

Which of the following statements is/are correct? (a) all real gases are less compressible

Select the correct statement : (a) The value of compressibility factor ' Z ' for H_2 gas is great

Compressibility factor - Wikipedia
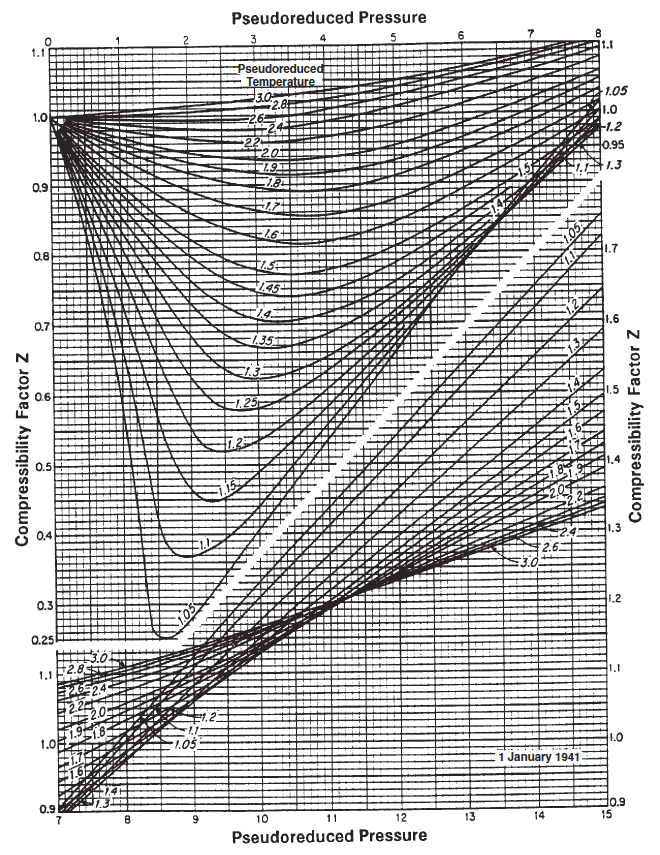
Real Gas Law - whitson wiki

If assertion is true but reason is false.

Real gas z-factor, as attributed to Standing and Katz, 9 plotted as a

The value of compressibility factor (`Z`) for an ideal gas is
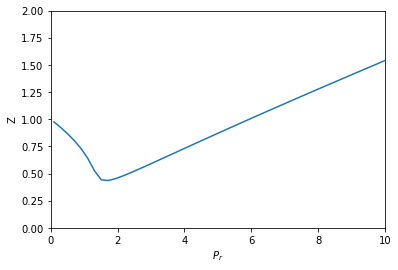
Compressibility factor variation from the van der Waals equation by three different approaches

Compressibility factor (z): real gases deviate from ideal behav-Turito

1. The compressibility factor, z, is the ratio of

Real Gas Behavior The Compression Factor (Z) [Example #2]
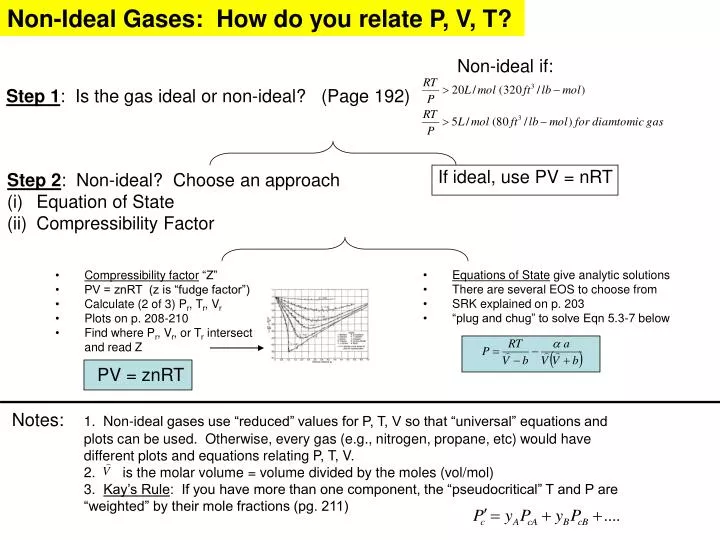
PPT - Step 1 : Is the gas ideal or non-ideal? (Page 192) PowerPoint Presentation - ID:3100094

At critical temperature, pressure and volume. The compressibility factor (Z) is 2

Solved The compressibility factor, Z, can be thought of as a