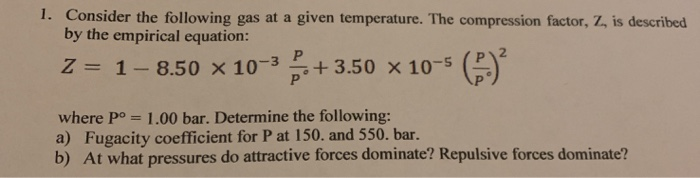the compression factor one mole of a vander waals gas 0 C and 100
Click here:point_up_2:to get an answer to your question :writing_hand:the compression factor for one mole of a vander waals gas at 0 c and
Click here👆to get an answer to your question ✍️ The compression factor one mole of a vander waals gas 0 C and 100 atm pressure is found to be 0-5
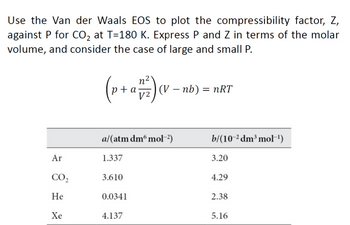
Answered: Use the Van der Waals EOS to plot the…

What is the compressibility factor Z for 0.02 mole of a van der waal's gas at pressure of 0.1 atm. Assume the size of gas molecule is negligible. Given: RT =20 L

SOLVED: Question 1) For water at 293 K and 1 atm, the isothermal compressibility (K) is 4.52 × 10-5 atm-1. If the pressure of exactly 1000 mL of water is increased from
The compression factor (compressibility factor) for one mole of a van der Waals' gas - Sarthaks eConnect

Djective lype Questions The compressibility factor of N2 moderate pressure range is equal to [where a & b are the van der Waal's constants] Pb Pb (2) 1- (1)RT RTV (3) (1RTV

18. The compressibility factor one mole of a vanderwaal's gas 0°C and 100 atm pressure is found to be 0.5. Assume that the volume of gas molecule is negligible calculate the vanderwaals
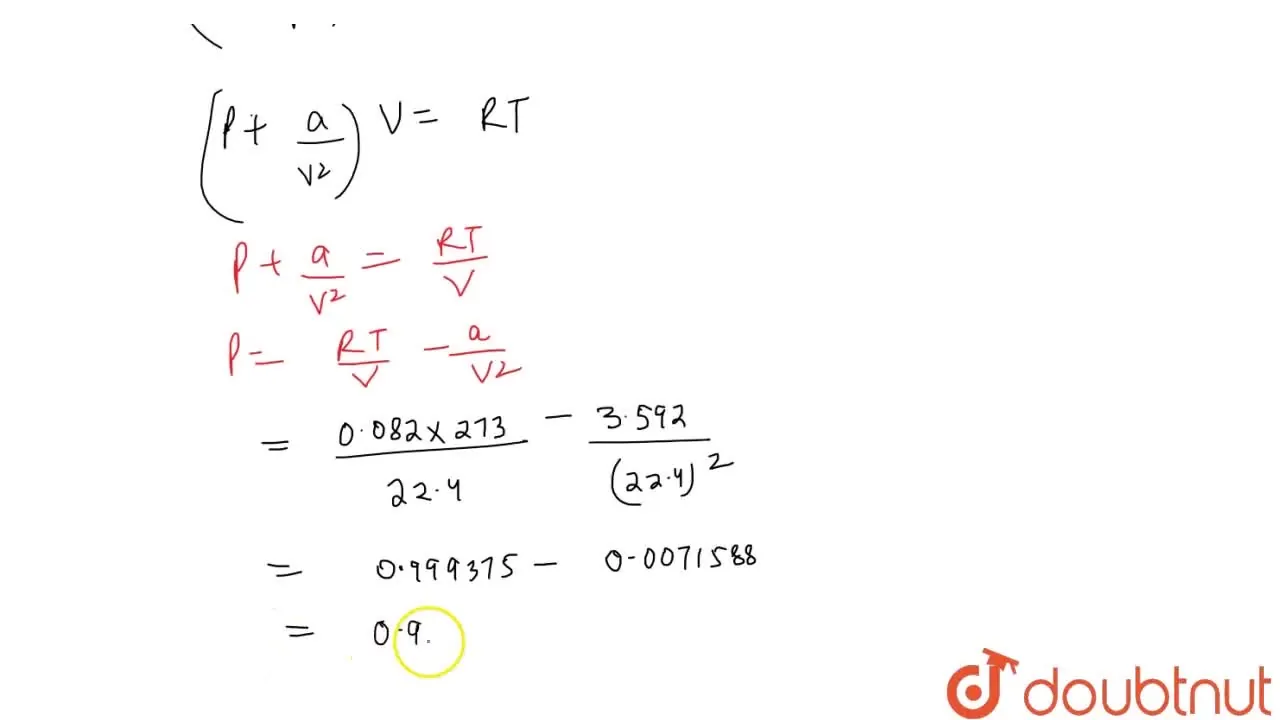
Calculate the pressure exerted by one mole of CO(2) gas at 273 K van d

The compression factor (compressibility factor) for one mole of a Van der..

The compression factor (compressibility factor) for 1 mol of a van der

Calculate the molar volume of argon at 100c and 100 atm on the assumption that it is a van der Waals

The compression factor (compressibility factor) one mole of a van der Waals' gas 0°C and 100 atm pressure is found to be 0.5. Assuming that the volume of a gas molecule is
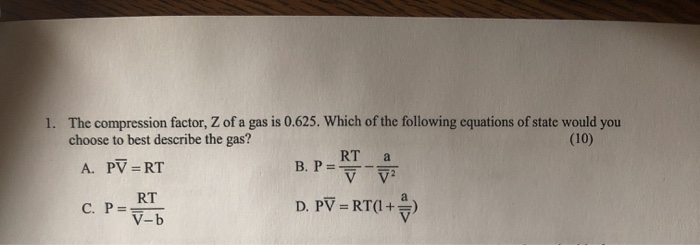
Solved 1. The compression factor, Z of a gas is 0.625. Which

At high pressure, the compressibility factor for one mole of van der w

The figure displays the plot of compression factor Z versus p a few gases. Which of the following statements is/are correct a van der Waals gas? HA The plot of I is